- Home
- EKG / ECG Machines
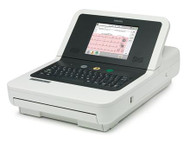
- Philips Pagewriter Trim III EKG W/ Cart "Refurbished"
Product Description
Fully Refurbished unit with 1 Year Warranty
Does your organization store ECGs in a central management system? Or do you network with cardiologists who overread ECGs and provide second opinions? Then PageWriter Trim III is for you. This proven performer supports the reporting, storage, and transmission of 12-lead ECG data using industry standard XML. You can connect directly to Philips TraceMasterVue ECG Management System. ECGs can be transmitted anywhere at anytime allowing you instant, secure access to patient reports.
- Easy-to-use features, such as a high-resolution 6.5-inch color screen
- Intuitive Patient Interface Module
- Multiple options to streamline information sharing including modem, USB, wired LAN or wireless 802.11 encrypted connectivity
- Adult and pediatric interpretation software built in. Also included is HP's Acute Cardiac Ischemia-Time Insensitive Predictive Instrument (ACI-TIPI) and Thrombolytic Predictive Instrument (TPI) applications.
Interpretive, with preview screen and full alphanumeric keyboard.
Specifications:
Physician Diagnostic Tools
- Reasons: Selectable explanations of interpretive statements
- Lead Placement Detection: Detects 19 different lead reversals
- Borderline Statement Suppression: Three configurable settings
Signal Processing
Patient Interface Module: Remote, microprocessor-controlled, digital module provides 5uV resolution
Display
- Size: 6.5 inch
- Colors: 64,000
Signal Quality Indicators
- Leads off advisory: Displays the label of any loose or disconnected leads/electrodes
- Lead Colors: Four
- Heart Rate: Continuous display of patient heart rate
- Print Preview: Full screen preview of waveforms prior to printing
Mechanical
- Dimensions: 388 x 310 x 176 mm (15.3 x 12.2 x 6.9 in)
- Weight (includes battery, patient module, lead wires, alligator clips, electrode pack and paper pack): 7.38 kg (16.3 lb)
Environmental
- Operating Conditions: 10 degrees to 40 degrees C (50-104 degrees F); 15% to 80% relative humidity (non-condensing); up to 4,550 m (15,000 ft.) altitude
- Storage Conditions: 0 degrees to 50 degrees C (32 degrees to 122 degrees F); 15% to 90% relative humidity (non-condensing); up to 4,550 m (15,000 ft.) altitude
Safety and Performance
International Standards and Regulations :
:
-
IEC 60601-1:1988 +A1:1991 +A2:1995 General Requirement for Safety
-
IEC 60601-1-2:2001 General Requirements for Safety for Electromagnetic Compatibility
-
IEC 60601-2-25:1993 + A1:1999 Safety of Electrocardiographs
-
IEC 60601-2-51:2003 Particular requirements for the safety and necessary performance of electrocardiographs
-
UL 2601-1:L1997 US General Requirements for Safety
-
CAN/CSA-C22.2 No. 601.1-M90 S1:1994 B:1996;AAMI EC11 1991:Diagnostic Electrocardiographic Devices
-
CISPR11:1997 + A1:1999 + A2:2002 Radio Frequency Disturbance, Limits and Methods of Test Group 1 Class B
-
JIST 1202:1998 Japanese Industrial Standard for Electrocardiographs
 Loading... Please wait...
Loading... Please wait...